Neurodegenerative Disease Resource Library
Explore examples of research, developmental milestones, and investigational treatment approaches targeting the central nervous system.
Filter
Huntington's Disease
Read an in-depth analysis of Huntington’s disease characteristics, diagnosis and management approaches, and genetic testing options.

AAV Gene Therapy Research Animation
Adeno-associated viral (AAV) vectors are designed to encapsulate genetic material for the potential treatment of genetic diseases. Explore the science behind AAV gene therapy research.
What Causes Genetic Disease?
Proteins carry out a variety of cellular and physiological functions in the body. See how changes in a person’s DNA can affect proteins and lead to genetic disease.
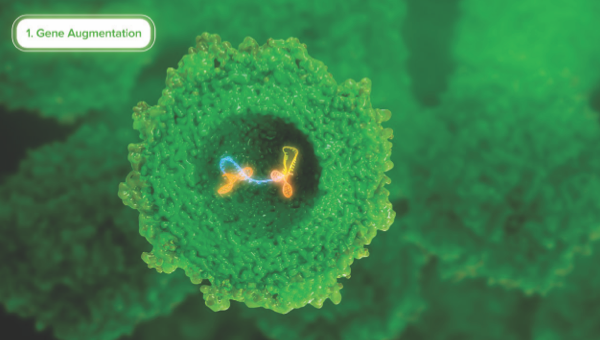
Gene Therapy Strategies
Learn how genetic therapy approaches can potentially correct genetic mutations to provide functional protein.
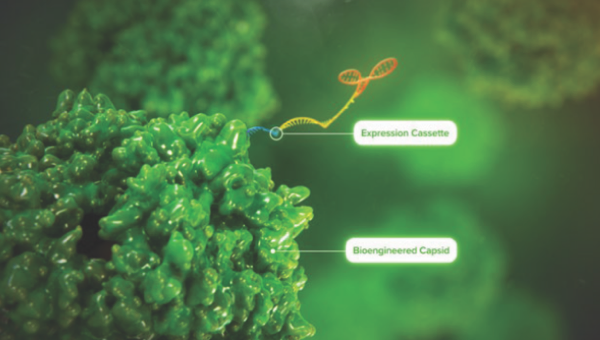
Biological Features of a Recombinant AAV
AAVs are commonly used as vectors in gene therapy. See how recombinant AAV vectors are designed and how they function in the body.
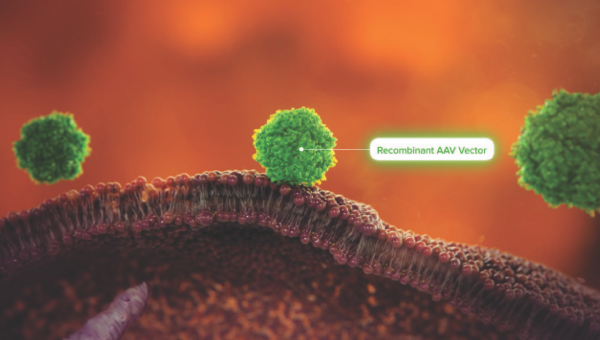
Targeted Cell AAV Delivery
Watch how recombinant AAV vectors delivered into the body locate specific surface receptors of target cells.

Delivery into Target Cells
DNA is delivered to the nucleus of the target cell to allow for gene expression of the relevant protein. Watch the process in action.

Gene Therapy and the Immune Response
Administering AAV vectors can activate different branches of the immune system. Learn about this process and research to develop immunosuppressive techniques.
Supplemental Resources
Explore additional neurodegenerative disease resources to supplement conversations with your patients.
Annual Neurology Events
At Spark Therapeutics, we’re striving to address unmet needs in rare genetic diseases. Here are some events relevant to our research.
Contact Spark® Therapeutics
Get information about Spark products and therapeutic areas or request a discussion with a medical science liaison.
Speak with a representative, Monday through Friday from 8 AM to 8 PM ET.




